40
Phase1
Finalists
10
Phase1
Winners
4C AIR, INC
Mask Name: BreSafeTM Transparent Mask. A semitransparency mask that provides excellent filtration and breathability utilizing patented nanotechnology.
Description: Our BreSafeTM transparent mask design provides clearer communication, improved filtration efficacy, and breathability with the ability to control the transparency level and filtration efficiency based on mask application.

Air Flo Labs, LLC
Mask Name: Flo Mask. Our Mission: Build the world's best face masks, period.
Description: Flo Mask was built upon three core pillars: tailored fit, comfort, and effectiveness. Using 3D scan data of real faces has allowed us to develop "the perfect” shape in creating a tailored fit respirator mask. A pillowy soft silicone gasket ensures physical comfort for long periods of use while a highly breathable filter allows for respiratory comfort. Our replaceable filters are made in the USA and have been Nelson Labs tested to filter over 99.8% of viruses. Flo Mask is currently used by thousands of kids across the USA and we're now focused on our adult version.

Air99, LLC
Mask Name: Airgami Mask. N95-grade mask with best-in-class breathability
Description: Airgami is an N95-grade mask with best-in-class breathability. The patented origami design creates a self-conforming tight fit. Airgami has a larger filter, which lowers breathing resistance and eliminates exhale valves. It is feather-light, cool, and comfortable to wear. It won't fog your glasses, won't fall off your nose, won't collapse onto your mouth, and won't muffle your voice. Multiple sizes and a micro-adjustable harness fit youth through adults. Airgami is reusable for up to a month and can be rinsed and disinfected in conventional or microwave ovens. Airgami protects against infected aerosols, wildfire smoke, air pollution, allergens and is ideal for front-line workers, travelers, seniors, children, educators, singers, and athletes.

Amazon.com Services, LLC
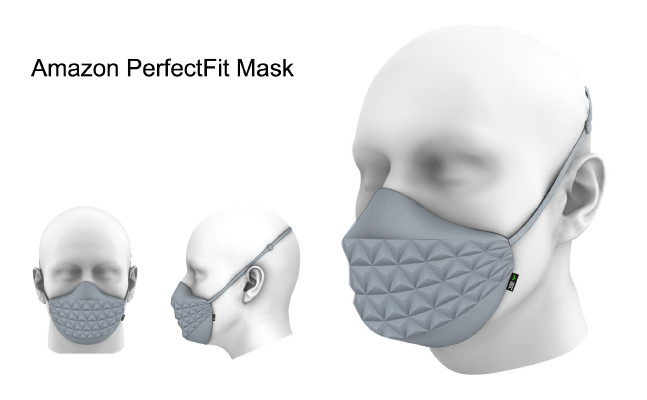
Mask Name: Amazon PerfectFit Mask
Description: The Amazon PerfectFit Mask is designed to protect users from inhaling pollutants and aerosols just like an N95 mask, but with the same comfort and style of a cloth mask or bandana. It has several innovative features, including a folded-paper "origami" design to improve breathability; 6 different sizes, enabling better fit to a wide variety of face shapes; soft, cooling fabrics with an adjustable headstrap or earloops to provide a gentle, tunable pressure against the face; and, finally, a range of inkjet-printable colors and patterns, making it feel more like apparel vs. a medical device.
AtmoBlue, Inc.
Mask Name: AtmoBlue v2
Description: We already provide comfortable safety with our existing powered air-purifying respirator, and in our next design, we are building an integrated sensor platform. The mask features HEPA filters and a customizable hard shell. The shell conceals a continuous strap giving the user a full range of motion and hides multiple fans and sensors. Finally, a silicone shell allows it to fit different face sizes. Tomorrow's mask will come with embedded technologies. We are building datadriven features that will let people know if they should change their filters, if they have a proper mask fit, and if they have co2 buildup.

Georgetown University
Mask Name: Reusable smart face masks using nanoporous metallic foams. Light as a feather
Description: The smart mask design utilizes nanoporous metal foams as the active filter media for efficient deep submicron particulate filtration. Their specially tailored nanostructures lead to comparable efficiencies and breathability as N95 face masks. The foams are light weight yet robust, can be easily cleaned and reused. They are also easy to reclaim and recycle. They can accommodate additional air cleaning mechanisms, such as electrostatics, catalytic reactions, and antimicrobial characteristics. The foams are made by scalable processes, thus are economically viable. They can be readily integrated with other mask designs, either as the filtration media themselves, or as inserts or cartridges.
Levi Strauss & Co.
Mask Name: Project: Veil. Using familiar tools and everyday skills to create a smart, simple, and effective solution for respiratory protection.
Description: Because the tools and skills we use to make a pair of Levi's constitute a globally standardized production capability, we've engineered the performance of an N95-equivalent respirator into a form that can be easily manufactured by any basic garment factory. Our simple solution activates the existing and underutilized apparel supply chain as an alternative resource to make high-performance, low-cost respirators - with no new equipment, training, or start-up costs. Our simplified approach to this challenge demonstrates that an effective respirator can be made anywhere there's a pair of scissors and a sewing machine.

PaciMask, LLC
Mask Name: PaciMask. It's just a mask, baby!
Description: The PaciMask is a face mask that incorporates a pacifier into a mask so that the child is able to enjoy the soothing effects of a pacifier while simultaneously wearing a mask. This increases the likelihood that this peer group will accept the idea of wearing a mask. The PaciMask supports the many different brands of pacifiers on the market. The pacifier is methodically affixed to the mask to ensure the accurate placement of the pacifier and secure fit of the mask. The mask is 100 percent cotton and is washable and reusable. The PaciMask includes fun prints and patterns, or favorite characters to further incentivize children to wear the mask.

RECm
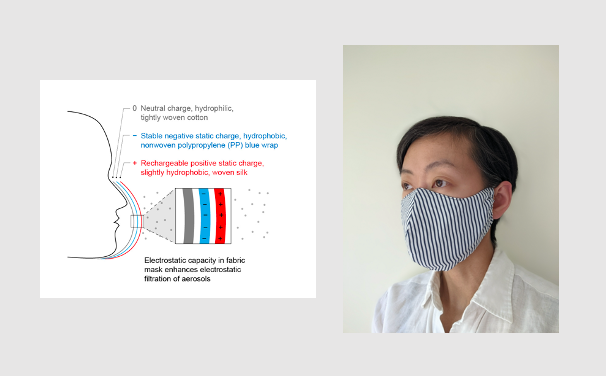
Mask Name: Rechargeable Electrostatic Capacity Mask (RECm). An everyday, washable, self-charging fabric mask with electrostatic filtration and fit similar to an N95
Description: The Rechargeable Electrostatic Capacity Mask (RECm) is made of 3 layers: silk, polypropylene filter, and cotton. RECm fits similar to an N95 in sizes for kids 2 and older, teens, and adults. After washing and drying, RECm can be easily recharged by using the triboelectric differential between silk and polypropylene: fold clean mask in half and rub for 20 seconds to regenerate electrostatic charge for enhanced aerosol filtration. The filter taps into millions of pounds of medical grade blue wrap discarded in good condition after single use sterilization. Establishing a safe protocol for reuse could improve and standardize filtration for all fabric masks.
The Makery Hilo
Mask Name: The Makery Mask. A safe and comfortable mask for everyone.
Description: Using widely available materials, the Makery Mask can be reproduced almost anywhere, even in one of the most remote population centers in the world: Hawai'i. Off-the-shelf materials does not mean our mask is compromising. We have developed proprietary ways to reuse medical sterile wraps and other spunblown materials and bind them permanently to a skin soft silicone mask that provides a leak-free seal to the face. Our innovation provides comfort, high filtration, an airspace for speaking clearly, a large surface filter for easy breathing, and we can even make customized masks within a few hours directly from iPhone12 lidar models.


