The Promise of Host-Based Therapeutics
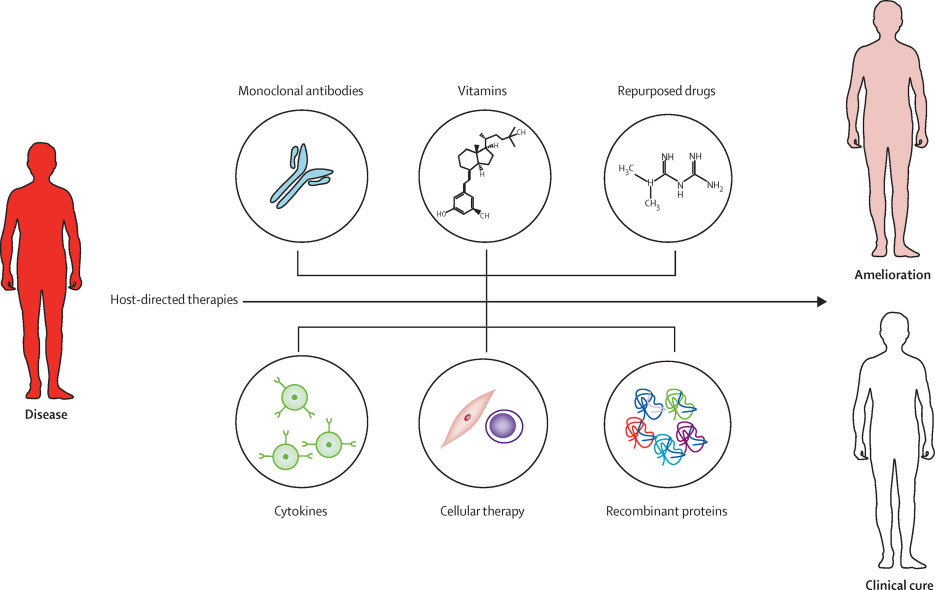
Host-directed therapeutics hold enormous potential to treat a wide array of medical conditions, from sepsis to chronic infections like tuberculosis and HIV and even cancer. What differentiates this class of therapies is both that it operates on a fundamentally different and potentially more effective level than traditional antivirals or antibiotics, and that the class creates unique opportunities for scale. We can be prepared for scenarios ranging from a chemical exposure event to almost any type of pandemic or outbreak.
Host-directed therapeutics are products that can help strengthen a person’s internal defense mechanisms against disease, decrease excess inflammation, or both, resulting in improved health outcomes such as less death, disability, or organ damage or even a complete recovery from the illness. Host-directed therapeutics don’t target a specific virus or bacteria, but rather try to modulate the body’s response in a way that provides an environment that is either unfavorable to the invader, or minimizes the body’s often pathological response to the insult. For example, in the case of sepsis, the body initiates a response to an infection that actually damages and even destroys its own tissues – such as excessive blood clotting (or thinning), shock, hyperinflammation, and diminished blood flow to the body’s vital organs including the brain, heart, and kidneys. This type of severe and overwhelming host response to an infection occurs is extremely difficult to reverse, which is one of the reasons why sepsis is so often a deadly illness. Even among patients who recovers from sepsis, people may be left with lifelong organ damage or other devastating and irreversible complications such as loss of limbs due to severe blood clots.
There are other types of harmful host responses that can serve as potential targets for host-directed therapies. In sepsis, for example, immune suppression due to immune cell apoptosis (cell death) can occur. This can include persistent immunosuppression, leading to secondary infections in later stages even months or years after infection, or a so-called “exhaustion” of lymphocytes. This response further contributes to inflammation, microvascular (small blood vessels) dysfunction, and organ failure as a long-term complication of sepsis. Additionally, during sepsis, a class of molecules known as caspases initiate a molecular cascade leading to cell death. Host reactions at the site of infection also can be problematic, such as the formation of granulomas (scar tissue) at the site of the infection; this type of problem occurs, for example with tuberculosis. Each one of these processes could be potential targets for future therapies.
Host-directed therapies are specifically useful against emerging pathogens in the critical interim period before specific pathogen-targeted therapies become available. In the setting of COVID-19, clinicians were evaluating numerous treatments that could address the pathologies associated with disease since direct-acting antivirals or neutralizing monoclonal antibodies were not available yet. Many of these interventions were host-directed; as an example, the use of dexamethasone to reduce inflammation saved lives in the setting of severe COVID. Anti-clotting medications such as heparin and enoxaparin were (and are) being utilized for hospitalized COVID-19 patients to decrease the need for ventilation and other organ support interventions, and even medicines such as ibuprofen could be helpful for decreasing inflammatory responses associated with COVID. These treatments were widely available in hospitals and repurposed to support patients’ innate defense mechanisms, in many cases before COVID-specific therapies emerged. However, randomized controlled trials (RCTs) are required early in the process to identify the most promising host-based treatment options in the event of future outbreaks. The discovery of additional pathogen-agnostic treatments for acute lung injury could assist in future respiratory pandemic preparedness.
U.S. Food and Drug Administration (FDA)-approved drugs can be repurposed for intervention early in the public health response in RCTs, on an Emergency Use Authorization (EUA) basis for use in treating illnesses like sepsis, or even treating injuries caused by Chemical, Biological, Radiological, and Nuclear (CBRN) agents. Exposures like this can happen due to terrorist attacks, industrial accidents, or transportation accidents and thus a rapid response is needed to minimize and treat these injuries. Host-directed therapeutics are also efficient in the breadth of potential diseases impacted – in contrast to therapies such as antiviral medications which are focused on limited types of viruses and target specific viral proteins. This efficiency is achieved by targeting common pathophysiologic processes between disparate diseases; for example, many anti-cancer host-directed therapies may also be useful for treating sepsis.
The current generation of host-directed therapeutics, however, are still rudimentary. Improved understanding of the complex dynamics of the immune system, along with advances in regenerative medicine and sensing, might allow for more sophisticated ways to precisely tune the immune system. Investing in next-generation host-directed therapeutics at BARDA's Division of Research, Innovation & Ventures (DRIVe), such as those fostered by the Solving Sepsis Program, can help us be better prepared to respond quickly and save lives even though we do not know what the next major outbreak, pandemic, or other incident may be.
For further information on funding and partnerships with BARDA DRIVe, please visit the Easy Broad Agency Announcement (EZ-BAA) System for Award Management (SAM.gov) page.
References
See footnotesZumla A, Rao M, Wallis RS, Kaufmann SH, Rustomjee R, Mwaba P, Vilaplana C, Yeboah-Manu D, Chakaya J, Ippolito G, Azhar E, Hoelscher M, Maeurer M; Host-Directed Therapies Network consortium. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis. 2016 Apr;16(4):e47-63. doi: 10.1016/S1473-3099(16)00078-5.
Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. Published January 22, 2021. https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients#:~:text=In%20large%20clinical%20trial%20conducted,the%20need%20for%20ventilation. Accessed May 8, 2021.
CDC – COVID-19 Guidance – Treatments Your Healthcare Provider Might Recommend if You Are Sick. https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html. August 9, 2021.
Kaufmann, S., Dorhoi, A., Hotchkiss, R. et al. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov 17, 35–56 (2018). https://doi.org/10.1038/nrd.2017.162