ImmuneChip+ is currently open.
For more information on this program, email ImmunechipBARDA@hhs.gov
OVERVIEW
Rapid development of medical countermeasures that mitigate pandemic and other CBRN threats is a key mission of BARDA. Microphysiological Systems (MPS) or “tissue chips” are 3D models of human and animal tissue that could be applied to significantly accelerate the discovery and validation of vaccines and therapeutics.
The ImmuneChip+ Program is developing MPS with fully integrated immune system components to reproduce the pathology of infectious disease and exposure to other CBRN threats. The ultimate goal of this program is to position new 3D tissue models that faithfully recapitulate components of the human body as an integral part of the medical countermeasure development process. This includes efforts in platform standardization of critical components, biosensors, characterization, scaling and control over multi-tissue systems and finally, application of manufacturing processes.
PARTNERS
Companies we have partnered with on this program to date
APPROACH
The ImmuneChip+ Program is developing MPS with fully integrated immune system components to reproduce the pathology of infectious disease and exposure to other CBRN threats. The ultimate goal of this program is to position new 3D tissue models that faithfully recapitulate components of the human body as an integral part of the medical countermeasure development process. This includes efforts in platform standardization of critical components, biosensors, characterization, scaling and control over multi-tissue systems and finally, application of manufacturing processes.
The ImmuneChip+ program aims to address key challenges facing commercialization of MPS technology, including:
- Immune system modeling
- Long-term tissue monitoring
- Integration of multiple tissue models
- Characterization of existing platforms and tissue models
Program Goals
Automated Manufacturing
Automated platform manufacturing and integration of biosensors.
Modular Tissue Platforms
Integration of multiple human tissue types anchored by an immune system model.
CBRN Insult
Model
Model of injury or disease due to exposure to a CBRN threat agent.
Platform Characterization
Biological evaluation of the MPS, recapitulation of existing clinical data.
Related Publications
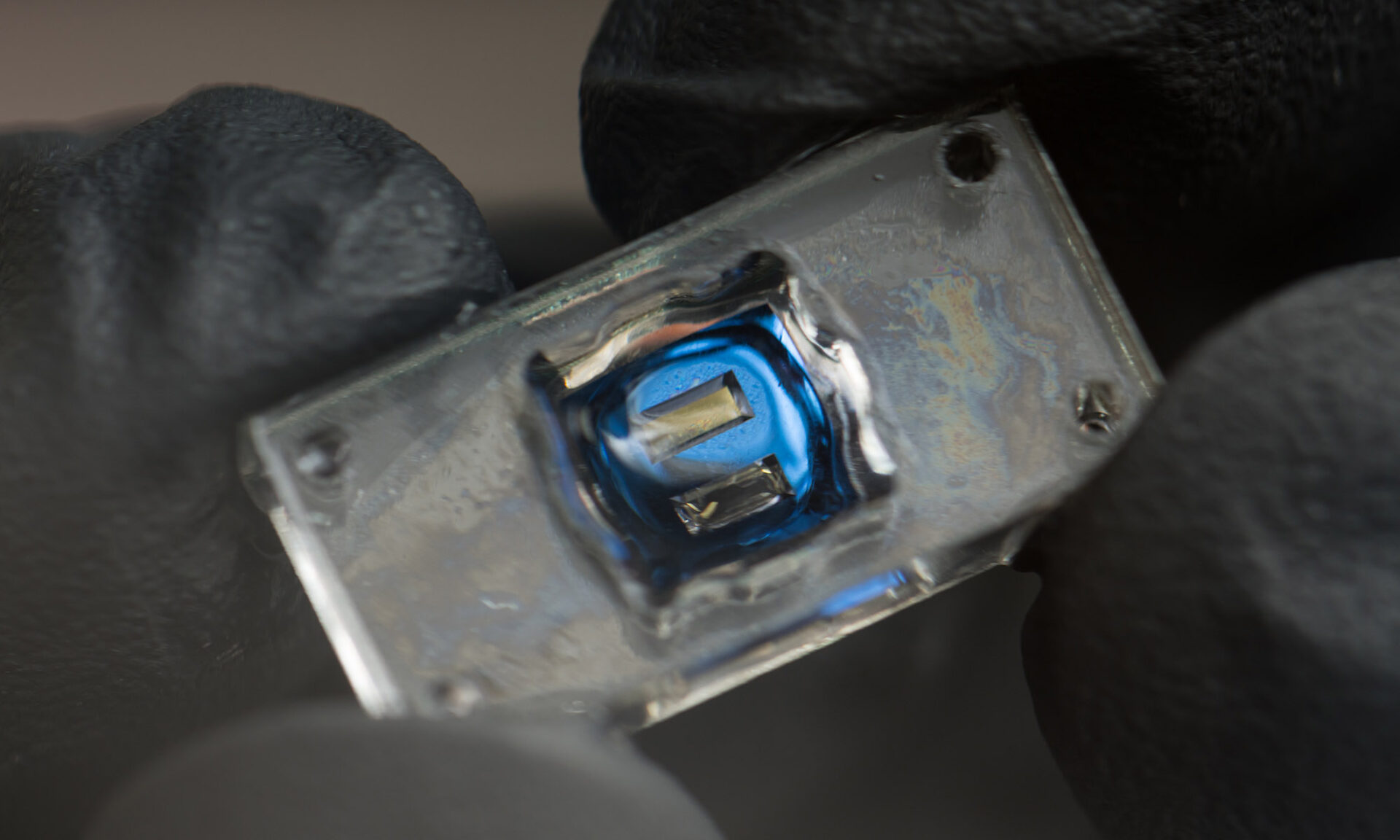
Scientists developing microchips with brain and lung tissue to study viral neuroinflammation
Scientists are developing advanced tools to understand and treat neurological symptoms such as brain fog associated with respiratory diseases like influenza. . .
ImmuneChip+ is currently open until September 28, 2028.