OVERVIEW
When health is compromised by infection, insult, or injury, the body's response can trigger a cascade of immune dysregulation and organ dysfunction, leading to poor patient outcomes. Treatments aimed at modulating these responses, known as host-directed therapeutics (HDTs), have the potential to prevent, reduce or mitigate immediate and long-term harm through promoting a balance of healthy cells, tissue, immune system or organ function.
PARTNERS
Companies we have partnered with on this program to date
APPROACH
HDTs by design target universal responses, representing a threat agnostic approach for a variety of health security threats, including pandemics. They offer a significant advantage for pandemic preparedness by eliminating the need for pathogen identification and pathogen specific treatment, which is a critical capability when facing an emerging or previously unknown pathogen. While HDTS may be used for the treatment of non-infectious conditions (e.g., high blood pressure, autoimmune disorders, oncology), they have not been widely applied to health security problems due to perceived challenges in development or ROI. To catalyze broader implementation of HDTs, we are supporting development of new or repurposed drugs, biologics, or devices.
Our current areas of HDT focus include:
- Interventions to prevent or reduce infection progression to severe outcomes
- Therapies that improve long term outcomes and prevent health deterioration
- Patient stratification to subtype populations for improved clinical management
Program Goals
Develop threat agnostic approaches for health security
Demonstrate the clinical utility of HDTs, in mitigating severe patient outcomes, (e.g. ARDS and sepsis)
Improve patient outcomes from home to hospital
Leverage HDTs to build resilience and restore homeostasis across the care continuum
Tailor therapeutic interventions to patients
Sub-type populations most likely to benefit from the HDT
HOST-DIRECTED THERAPEUTICS
AOI #18: Host-Directed Therapeutics
Funding
Opened
10/18/22
Amendment
#1
Funding
Closes
6/30/23
Related Publications And Presentations

Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012-2018
To provide contemporary estimates of the burdens (costs and mortality) associated with acute inpatient Medicare beneficiary admissions for sepsis.
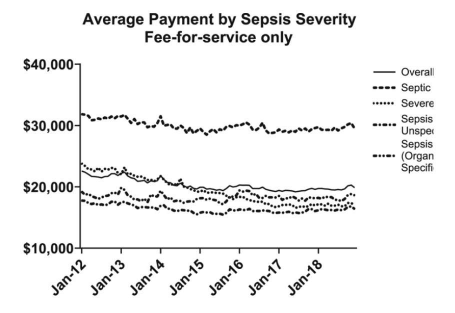
Sepsis Among Medicare Beneficiaries: 2. The Trajectories of Sepsis, 2012-2018
To distinguish characteristics of Medicare beneficiaries who will have an acute inpatient admission for sepsis from those who have an inpatient admission without sepsis, and to describe their further trajectories during and subsequent

Sepsis Among Medicare Beneficiaries: 3. The Methods, Models, and Forecasts of Sepsis, 2012-2018
To evaluate the impact of sepsis, age, and comorbidities on death following an acute inpatient admission and to model and forecast inpatient and skilled nursing facility costs for Medicare beneficiaries during and
Sepsis Among Medicare Beneficiaries: 4. Precoronavirus Disease 2019 Update January 2012 - February 2020
To provide updated information on the burdens of sepsis during acute inpatient admissions for Medicare beneficiaries.

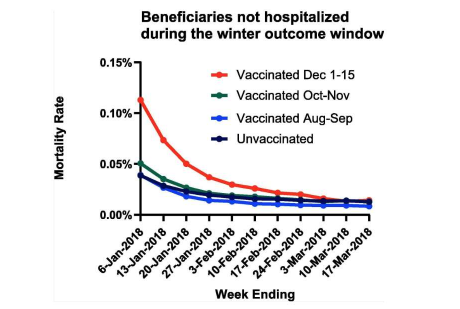
Seasonal influenza vaccination is associated with reduced risk of death among Medicare beneficiaries
Influenza causes substantial mortality, especially among older persons. Influenza vaccines are rarely more than 50% effective and rarely reach more than half of the US Medicare population...
Applying lessons learned from COVID-19 therapeutic trials to improve future ALI/ARDS trials
Host-directed therapeutics targeting immune dysregulation are considered the most promising approach to address the unmet clinical need for acute lung injury...
Program Faq
Our technology addresses either a bacterial or a viral infection. Would the HDT program be interested?
For this program, the medical countermeasure should be able to address infection via any etiology, and is not limited to one or the other.
We have developed a trial plan for the technology, but do not have any clinical data. Is this sufficient for proposal submission for a HDT AOI?
Proposals must include prior demonstration and preliminary clinical data to support the use for their intended indication. Additionally, the device should be considered near-translational science, and these AOls are unable to fund exploratory efforts at this time.
Can the HDT program help us plan and tailor our proposed clinical study?
We are unable to inform or guide companies on what specific test models to use to collect clinical data.