OVERVIEW
DRIVe is seeking proposals to develop novel instrumentation-based platform technologies for on-demand, simultaneous detection of multiple biochemical health markers at the point of need. The goal is to obtain quantitative information about patients’ health status at the point of need, avoiding the long sample-to-answer times and personnel training requirements of traditional central laboratory testing that can lead to delays in receiving care.
PARTNERS
Companies we have partnered with on this program to date
APPROACH
Support the development and validation of new platform technologies for simple, rapid multiplexed analysis of biological specimens in the home setting.
These novel technologies:
- Use non-invasive and minimally invasive samples to provide quantitative information about a patient's health status
- Are readily adaptable to a broad menu of test panels to cover a wide range of disease states as well as standard health assessments
- Offer rapid results for interpretation by health care providers
Program Goals
New Analytical Solutions
Novel biomarker sensing and readout technologies that enable quantitation
Flexible Solutions
Platform technologies that are adaptable to a broad menu of multiplex test panels
New Biological Samples
Detection of host biomarkers in novel sample types: interstitial fluid, sweat, saliva, breath
LAB AT HOME
Funding
Opened
8/25/21
Amendment
#14
Revised AOI
Description
1/7/2022
Amendment
#18
New
Amendment
10/22/2022
Amendment
#001New
Amendment
2/1/23
Amendment
#006New
Amendment
9/13/23
Amendment
#012New
Amendment
12/14/2023
Amendment
#18
Extended
closing date
3/12/2024
Amendment
#26
New
Amendment
5/9/2024
Amendment
#29
Funding
Closes
5/15/24
Related Publications And Presentations
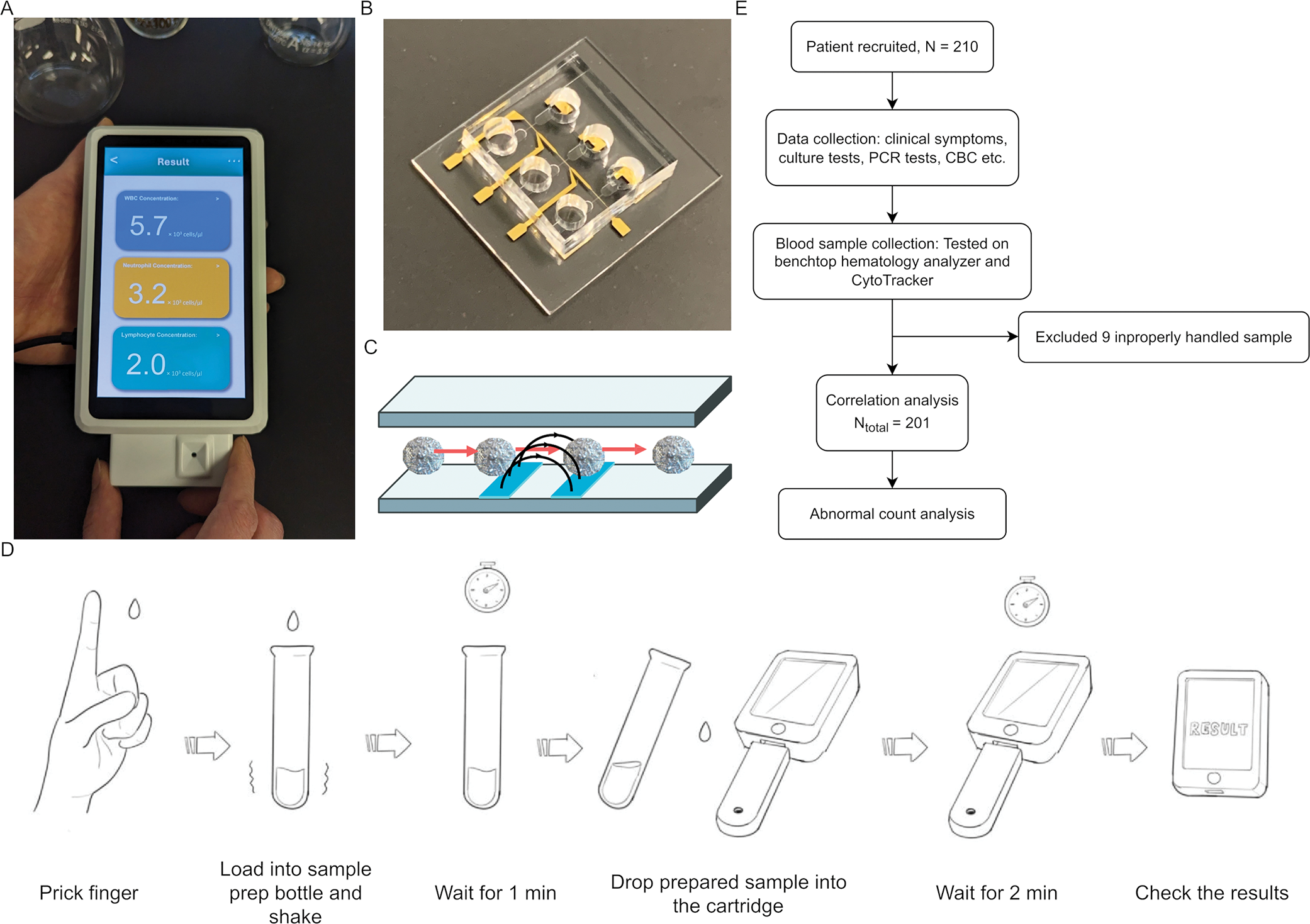
Clinical evaluation of a fully electronic microfluidic white blood cell analyzer
The White Blood Cell (WBC) count is one of the key parameters signaling the health of the immune system. Abnormal WBC counts often signal a systemic insult to the body…
Looking for General FAQ?
Lab at Home is paused after May 15, 2024