OVERVIEW
Rapid diagnosis and treatment of infectious diseases are critical to both the patient and the broader community. ENACT (Early Notification to Act, Control, and Treat) partners with innovators to develop disruptive technologies that detect infection, prognosticate outcomes, and enable early intervention following exposures to both natural and man-made infectious threats.
PARTNERS
Companies we have partnered with on this program to date
APPROACH
The programmatic vision is to upend the traditional doctor-patient paradigm by empowering patients, health-care providers, and public health organizations with early actionable health status information.
To this end, ENACT supports:
- Research and development of novel physiological sensors
- Data analytics platforms to collect and interpret physiological data
- Algorithms capable of Identifying any major health status changes
Program Goals
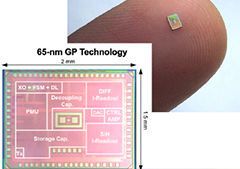
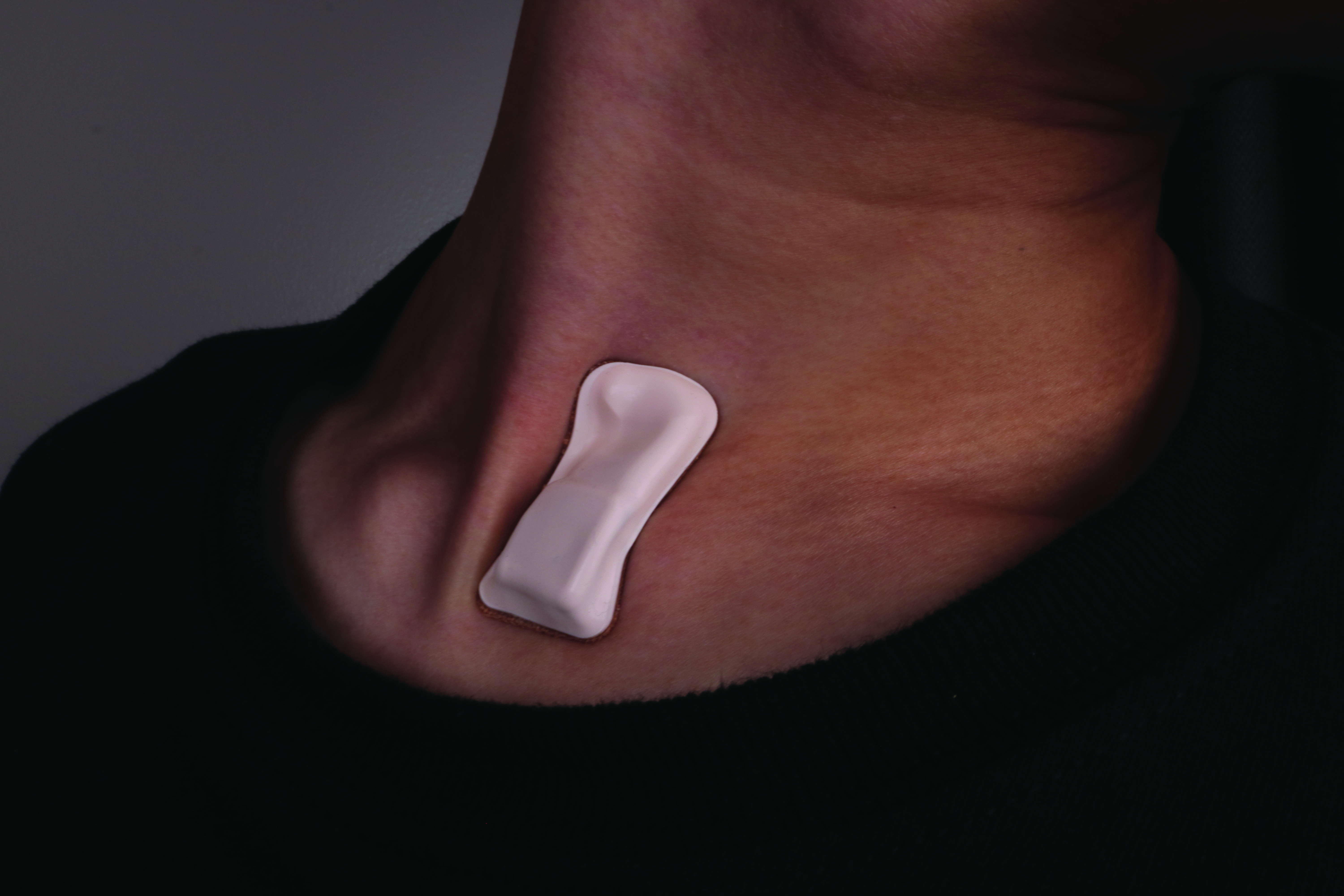
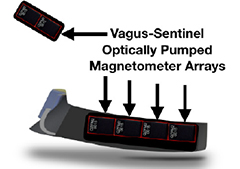
Develop Wearable Sensors
Capable of monitoring physiological changes from the user's baseline
Identify Robust Algorithms
Analyze the resulting clinical data and detect health and illness signatures in individuals
Identify Key Markers of Health Status
Enable high-accuracy pre-symptomatic detection of infection

enact
Funding
Opened
2/3/20
Amendment
#1
Funding
Closed
8/25/21
Related Publications

Ultrasound Neuromodulation of the Spleen Has TimeDependent AntiInflammatory Effect in a Pneumonia Model
Interfaces between the nervous and immune systems have been shown essential for the coordination and regulation of immune responses. Non-invasive ultrasound stimulation...

Accelerated electron transfer in nanostructured electrodes improves the sensitivity of electrochemical biosensors
Electrochemical biosensors hold the exciting potential to integrate molecular detection with signal processing and wireless communication in a miniaturized, low-cost system...
An Adhesive-Integrated Stretchable Silver Chloride Electrode Array for Unobtrusive Monitoring of Gastric Neuromuscular
Here, an unobtrusive, adhesive integrated electrode array for continuous monitoring of stomach electric activity is introduced. This patient-friendly, disposable peel and-stick adhesive device...
Program Faq
May an institution submit more than one proposal on behalf of investigators/teams? Is there a limit to the number of proposals that may be submitted by an institution?
An institution is currently allowed to submit more than one abstract on behalf of investigators, or teams. There is currently no limit to the number of abstracts received by an offeror. It is recommended that an offeror submit only their best products for consideration with the potential to radically transform Health Security.
Is follow-on funding available once ENACT funded EZ BAA projects are completed?
Projects completed under ENACT funding are NOT guaranteed additional funding from any government source (including ENACT).
Can ENACT identify partners for us to complete the work envisioned?
No. The government does not suggest or requiring any specific partnerships and will not provide match-making services.
Does ENACT fund diagnostics?
At this time host-based diagnostics are considered, but pathogen detection technologies are not considered to be within scope. Host based platforms should be targeted for use as wearables or athome, and should not be designed specifically for use in doctor's offices or clinics.
What stage of technology development does ENACT consider?
While there is no required starting TRL (technology readiness level), it is preferred that projects are close to ready for commercialization by the end of the funding period.
Is FDA engagement required for ENACT projects?
While FDA approval is typically not required by end of project, engagement with FDA typically is, but this depends on the proposed project.
Are templates of successful ENACT abstracts available?
ENACT cannot provide sample abstracts.
Can you submit more than once and on multiple targets?
Yes, potential partners are free to submit more than one abstract so long as those projects are discrete, stand-alone projects.
What resources are available to applicants in technical and business development?
Applicants are welcome to consult with DRIVe's Accelerator network, which provides wraparound technical and business development support services.