DRIVE MISSION
To enhance BARDA's preparedness for and response to dynamic or unpredictable threats by fostering resilient health security ecosystems and enabling the development and adoption of innovative medical countermeasures and related capabilities.
DRIVE VISION
To ensure the most effective life-saving medical countermeasures are developed for, available to, and used by everyone who needs them when they need them.
For General Inquiries : drive@hhs.gov
Unique public-private partnerships, new authorities, and an ecosystem of restless innovation.
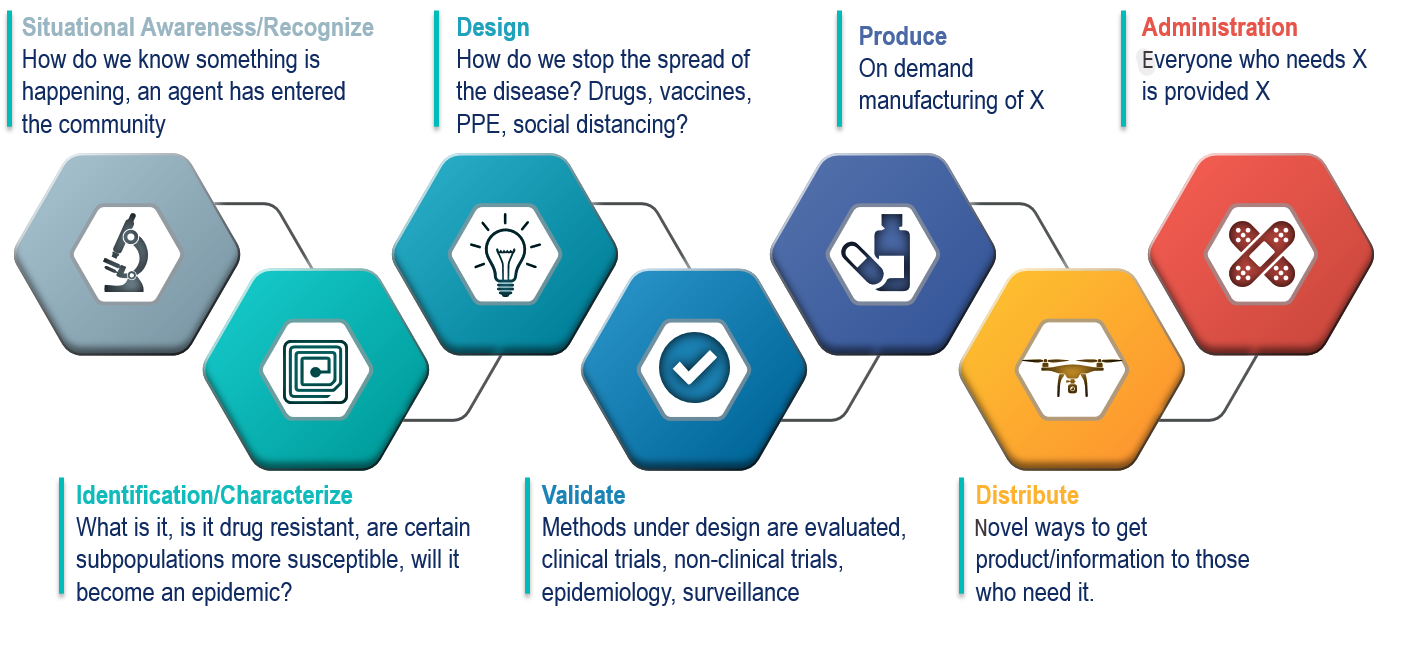
The Drive Business Model
DRIVe is forming unique public-private partnerships. DRIVe will leverage new authorities given to BARDA under the 21st Century Cures Act to stimulate innovation through both non-dilutive funding and dilutive funding. In addition, through our DRIVe Accelerator Network, we will have teams forward deployed throughout the USA to identify promising solutions wherever innovation is happening. Together we are building out an ecosystem of restless innovation, driven by industry and the entrepreneurial community, to address our nation's largest health security threats.